


Harnessing the power of data and advanced analytics, Novartis set out to redefine how pharmaceutical research addresses patient safety and drug innovation. Collaborating on an AI-driven initiative, DevsData LLC’s team developed a solution that detects adverse drug reactions 60% faster. The project shows how artificial intelligence can accelerate progress in one of the world’s most regulated and data-intensive industries.
Novartis is a Swiss multinational healthcare company headquartered in Basel, Switzerland, known for its focus on scientific innovation and data-driven medicine. The company develops therapies across oncology, immunology, neuroscience, and cardiovascular care, combining advanced research with digital technologies to redefine modern treatment development.
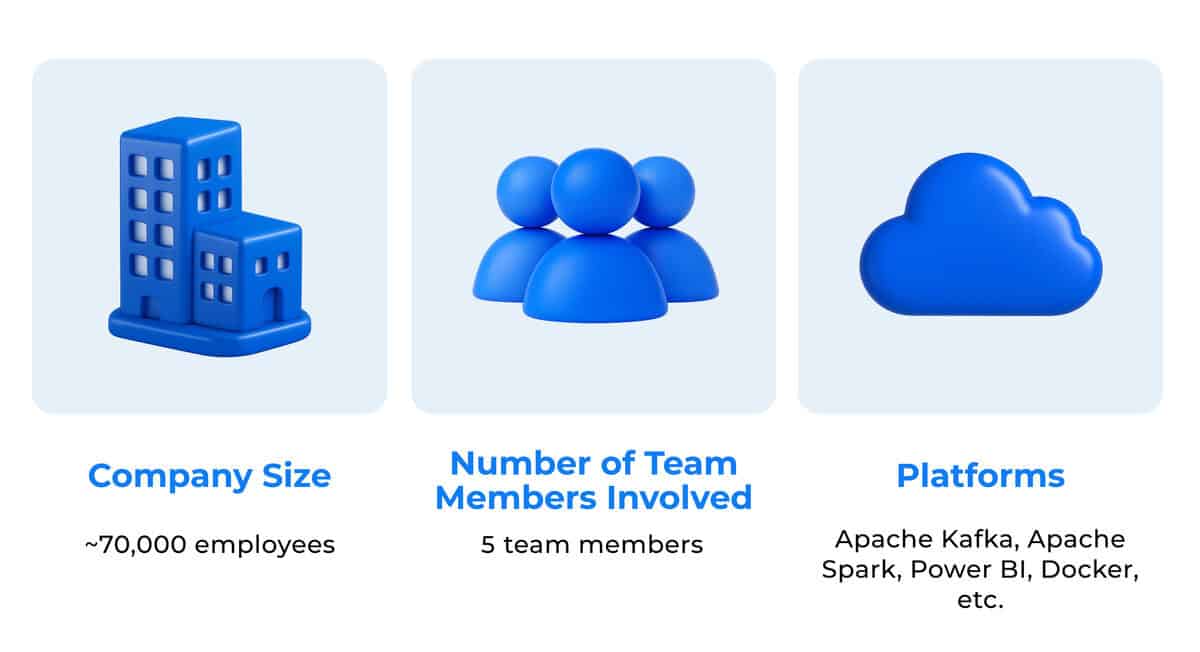
In 2024, Novartis reported $50.3 billion in annual revenue, representing a 12% year-over-year increase, reflecting both strong product performance and strategic growth across key markets. Employing over 70000 people and operating in more than 140 countries, the company supports research and clinical activity on a global scale, enabling faster, more coordinated therapeutic delivery across healthcare systems.
The organization’s recent efforts have centered on leveraging artificial intelligence and big data analytics to accelerate drug discovery and improve patient safety. Through collaborations with leading technology partners and academic institutions, Novartis has positioned itself at the intersection of life sciences and digital innovation, using AI to optimize clinical trial design, analyze patient data, and monitor drug safety post-launch.
Highlights:
As a global pharmaceutical enterprise, Novartis manages vast volumes of clinical and patient data. Monitoring adverse drug reactions (ADRs) after market release had become a growing challenge, as traditional pharmacovigilance systems were limited to formal reporting channels, increasing the risk of delayed safety signal detection and slower regulatory response. Increasingly, patients were sharing experiences on social media and forums – data that remained largely untapped. The inability to process this unstructured information in real time created a risk of delayed ADR detection, potentially affecting patient safety and regulatory compliance.
Despite having robust internal data science capabilities, Novartis’s in-house systems were not optimized for large-scale social media monitoring and natural language analysis. The company needed a solution that could accurately filter irrelevant content, extract key entities such as drug names and symptoms, and integrate insights into existing drug safety workflows. Developing such an advanced NLP-driven system internally would have required up to 12 months of experimentation and substantial resources, delaying critical improvements in post-market surveillance.
Recognizing the urgency and complexity of the task, Novartis partnered with DevsData LLC to accelerate the process. The collaboration combined Novartis’s pharmaceutical expertise with DevsData LLC’s specialization in machine learning, big data processing, and custom AI solution development. The partnership formed around a focused objective: to design and implement a system capable of scanning, classifying, and analyzing vast data streams from social networks and public platforms. Achieving this required the kind of scalable data engineering and domain-specific NLP expertise that DevsData LLC could deliver, based on previous experience on NLP and Al projects, enabling earlier, data-driven detection of potential drug-related safety issues worldwide.
The partnership aimed to design an AI-powered system capable of detecting adverse drug reactions faster and more accurately, transforming how Novartis monitors patient safety.
From the outset, DevsData LLC framed the engagement around extracting strategic insight from data rather than developing technology in isolation. The goal was to design a scalable and compliant analytical framework capable of detecting early signs of harmful drug responses from vast, unstructured online data sources, which is critical in pharma environments where scalability and regulatory compliance must be built in from day one. Drawing on our prior experience with complex, data-intensive environments, such as our AI and data science engagement with Maersk Tankers, where we delivered secure, production-ready analytics models under strict confidentiality, we emphasized modular architecture, transparency, and full alignment with internal governance frameworks from the start.
Together with Novartis’s pharmacovigilance experts, we established the core success criteria: accuracy in ADR identification, interpretability of results for clinical teams, and seamless integration into existing workflows. To achieve these goals efficiently, we proposed a phased delivery model, beginning with conceptual validation, followed by iterative model refinement and progressive scaling into production.
Below, we outline the objectives step-by-step:
| Strategic pillar | Description |
|---|---|
| Scalability | Designed a modular framework capable of processing millions of real-world data points in real time. |
| Compliance | Ensured full alignment with pharmaceutical data protection standards and internal governance protocols. |
| Accuracy | Applied domain-specific methodologies to improve the precision of ADR detection and reduce false positives. |
| Integration | Planned seamless interoperability with Novartis’s existing pharmacovigilance and analytics infrastructure. |
| Iterative delivery | Adopted a phased model: prototype, validate, scale – to balance innovation with operational reliability. |
The DevsData LLC team assigned to the project was composed of five specialists: two data scientists, two ML engineers, and a backend developer, selected for their combined expertise in large-scale data processing, AI model deployment, and system reliability. The compact structure was deliberately chosen to maintain agility while ensuring all core components of the solution lifecycle were covered. This approach drew on DevsData LLC’s diverse pool of specialists across data modeling, machine learning, and backend integration, enabling the team to match the project’s technical breadth without compromising delivery speed.
Collaboration with the client was organized through a structured, cross-functional workflow. The DevsData team worked closely with stakeholders from Novartis’s data science, digital innovation, and medical insights functions, ensuring that technical development remained aligned with business and research objectives. Regular alignment calls were held to validate assumptions, review interim findings, and adjust priorities, while asynchronous communication channels were used to resolve technical questions efficiently. Compliance and data governance stakeholders were also involved at key checkpoints to ensure that data handling, model behavior, and system access met internal regulatory standards.
The team leveraged technologies such as Python, TensorFlow, and FastAPI, along with cloud-based infrastructure for secure data handling and model scalability. This setup ensured rapid iteration, efficient resource allocation, and full alignment with Novartis’s technical and compliance requirements.
Do you have IT outsourcing needs?
The implementation phase brought DevsData LLC’s strategic plan into practice. A dedicated team was deployed within Novartis’s secure environment to build the full analytical pipeline. The execution process was structured into clearly defined stages:
The following diagram illustrates the four-stage workflow we implemented:
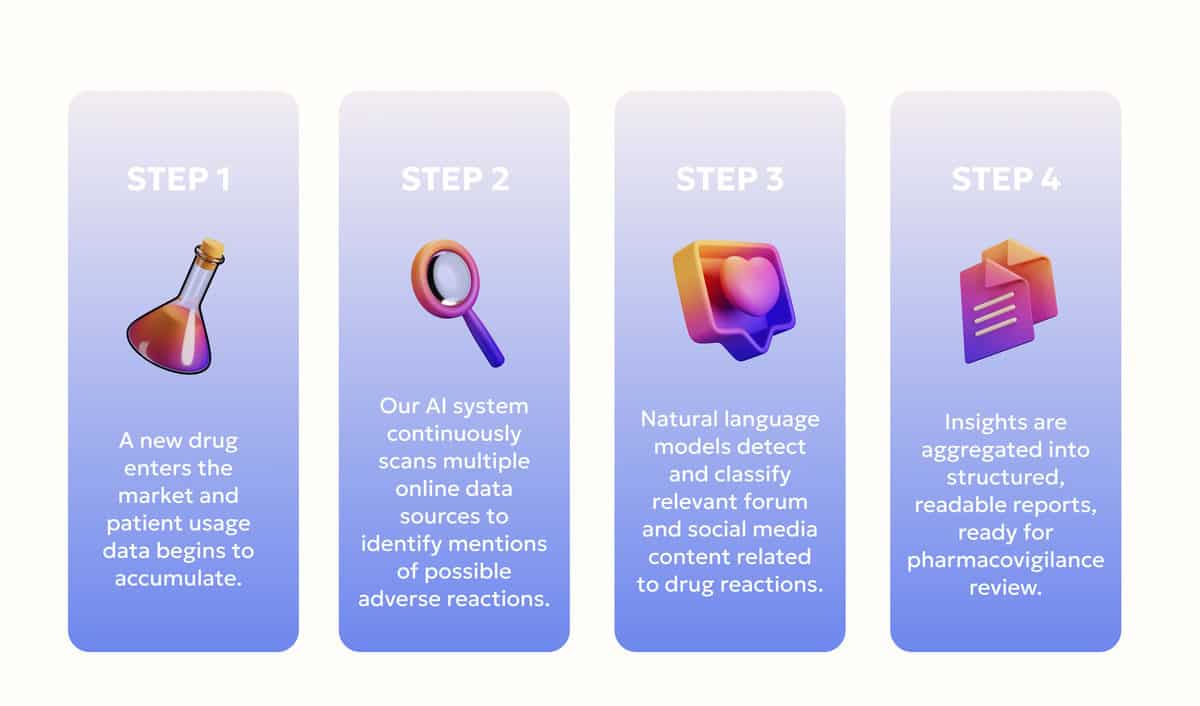
To ensure scalability and security, DevsData LLC relied on a robust technology stack that included Apache Kafka for real-time data streaming, Apache Spark for large-scale data processing, and Hugging Face Transformers combined with spaCy for natural language analysis. Power BI was used to visualize ADR trends and generate human-readable dashboards accessible to Novartis analysts. All environments were containerized with Docker and deployed within Novartis’s internal cloud infrastructure to maintain full compliance with data governance protocols.
Our prior work with Maersk Tankers proved invaluable here, particularly the experience of operating under strict confidentiality, designing modular pipelines, and embedding external specialists into secure client ecosystems. Leveraging these practices, DevsData LLC achieved rapid integration with Novartis’s teams and maintained delivery velocity without compromising data protection.
The final system was delivered as a production-ready, scalable platform complete with documentation, and knowledge transfer sessions, enabling Novartis to manage and evolve the solution internally.
Within a four-month engagement, DevsData LLC successfully designed, implemented, and deployed a production-ready pharmacovigilance solution for Novartis. The system significantly improved the speed and accuracy of detecting adverse drug reactions (ADRs) across social media and public data sources. Early results demonstrated that potential ADR signals could be surfaced up to 60% faster compared to traditional manual monitoring, helping Novartis identify safety issues at earlier stages and reduce overall reporting latency.
The solution’s architecture enabled high scalability and real-time processing without disrupting existing workflows. Internal Novartis teams reported smoother data-flow integration, reduced manual review workload, and enhanced visibility into patient safety trends across multiple markets. These improvements also contributed to better resource allocation, faster return on investment, and quicker decision-making within pharmacovigilance units.
| Impact area | Result |
|---|---|
| Delivery timeframe | Full system delivered and validated within 4 months |
| Detection efficiency | 60% faster ADR identification compared to baseline methods |
| Operational gains | Reduced manual data review time by 40% |
| Scalability | Capable of processing millions of public data entries in real time |
The engagement reinforced Novartis’s commitment to innovation through AI-driven safety analytics while showcasing DevsData LLC’s ability to deliver tangible, domain-specific results in a highly regulated environment.
Within just four months, the DevsData solution accelerated ADR detection by 60%, proving how data-driven innovation can reshape pharmacovigilance at a global scale.
Following the project’s success, discussions have centered on expanding the system’s analytical scope beyond adverse drug reactions to include predictive patient-safety modeling and treatment-outcome analysis, enabling earlier detection of emerging risks, more informed clinical decisions, and proactive intervention before issues escalate. Future collaboration will focus on incorporating multilingual sentiment analytics, connecting the platform with internal clinical data streams, and developing visualization dashboards for real-time regulatory reporting, expanding global pharmacovigilance coverage by capturing safety signals across regions, languages, and patient populations that are typically underrepresented in traditional reporting systems.
DevsData LLC continues to serve as Novartis’s strategic technology partner, supporting ongoing model optimization and infrastructure scaling. The next phase aims to build on the foundation established during the initial engagement, transforming the pharmacovigilance tool into a broader AI ecosystem capable of driving innovation across Novartis’s R&D, clinical, and post-market surveillance operations.
Exploring AI solutions for large-scale data environments?
Schedule a consultation with DevsData LLC to discuss how advanced analytics and machine learning can strengthen your organization’s data monitoring, compliance, and decision-making capabilities. Reach out to us at [email protected] or visit our website at www.devsdata.com.
DevsData – your premium technology partner
DevsData is a boutique tech recruitment and software agency. Develop your software project with veteran engineers or scale up an in-house tech team of developers with relevant industry experience.
Free consultation with a software expert
🎧 Schedule a meeting
FEATURED IN


DevsData LLC is truly exceptional – their backend developers are some of the best I’ve ever worked with.”
Nicholas Johnson
Mentor at YC, serial entrepreneur





Categories: Big data, data analytics | Software and technology | IT recruitment blog | IT in Poland | Content hub (blog)
